Home
Tutorial's
Image collection
Calculator
Projects
MCQ's
3d Models
Invention Hub
How it works
Download Our app
Module 1: Electricity
Everyone knows what matter is, but the important thing is to understand how science uses it. Everything that is heavy and requires space, like water, copper, wood, etc., is referred to as matter.An atom is basically composed of a nucleus and electrons that orbit or rotate around it in different directions, as seen in the image below.
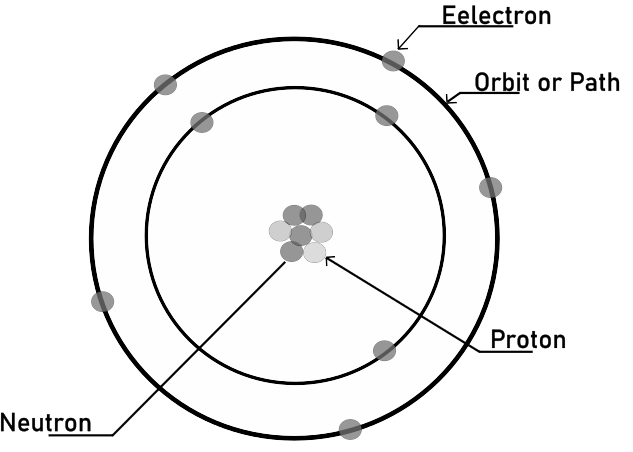
Protons, which have a positive charge, make up the nucleus, whereas neutrons have no charge. The charge of the protons is +1.602 x 10-19 coulombs. The mass of an electron is 9.1 x 10-31 while that of a proton is 1.66 x 10-27. Since the proton has a mass 1837 times that of the electron, the electron is considered to be light in weight, and the electrons are positioned in various orbits.
The electrons at the outermost orbits are referred to as valency electrons; these electrons are easily withdrawn and loosely bound, particularly for copper and aluminum. These electrons travel randomly between atoms, but when a potential difference, pressure, or vibration is applied to it, all of the free electrons move in the same direction.
Electric current is the passage of electrons in a conductor. However, because the electrons in an insulator are tightly bonded to the nucleus, any pressure applied will cause it to remain unchanged. Protons, which have a positive charge, make up the nucleus, whereas neutrons have no charge. The charge of the protons is +1.602 x 10-19 coulombs. The mass of an electron is 9.1 x 10-31 while that of a proton is 1.66 x 10-27. Since the proton has a mass 1837 times that of the electron, the electron is considered to be light in weight, and the electrons are positioned in various orbits.
The electrons at the outermost orbits are referred to as valency electrons; these electrons are easily withdrawn and loosely bounded, particularly for copper and aluminum. These electrons travel randomly between atoms, but when a potential difference, pressure, or vibration is applied to it, all of the free electrons move in the same direction. Electric current is the passage of electrons in a conductor. However, because the electrons in an insulator are tightly bonded to the nucleus, any pressure applied will cause it to remain unchanged.
Related articles
